
COLLECT DATA DIRECTLY FROM PATIENTS AT THE RIGHT MOMENT
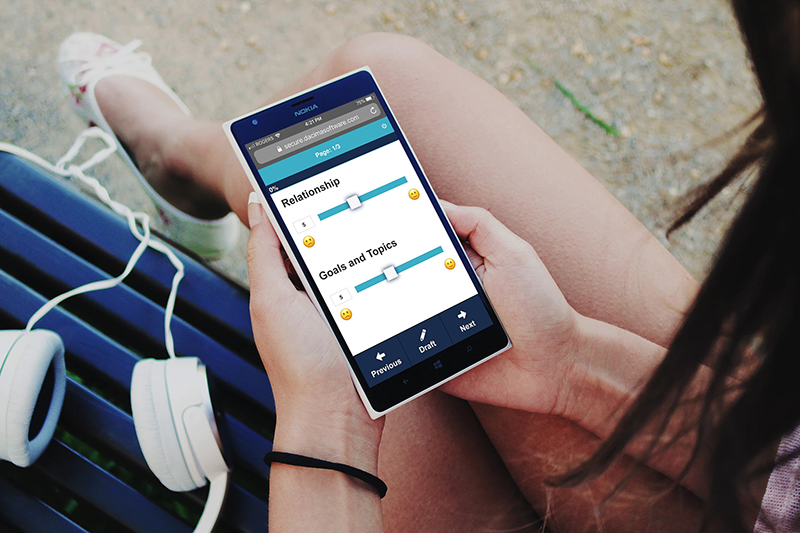
EvidentIQ eDiary offers an intuitive web interface that simplifies diary data collection compared to traditional paper-based diaries and enhances patient engagement.

eDIARY
IMPROVE PATIENT ENGAGEMENT AND DATA QUALITY
EvidentIQ eDiary is an electronic Clinical Outcome Assessment (eCOA) software that allows you to build online diaries to be filled out by participants using a tablet or smartphone. Our eDiary complies with FDA 21 CFR Part 11, Good Clinical Practices (GCP) and contains a complete audit trail, making it the best choice for medical and health-related research studies.
FEATURES
I DONT KNOW YET
Powerful Web Database Development
Allows you to develop databases without the need for computer programming expertise and access them through any web browser independent of the operating system.
Flexible, Commercial Technology
Activating or deactivating features, the software can be easily tailored for different research study designs, such as randomized clinical trials, observational epidemiological studies, patient registries, patient interviews or administrative databases.
Powerful Web Database Development
Allows you to develop databases without the need for computer programming expertise and access them through any web browser independent of the operating system.
Flexible, Commercial Technology
Activating or deactivating features, the software can be easily tailored for different research study designs, such as randomized clinical trials, observational epidemiological studies, patient registries, patient interviews or administrative databases.

DIGITAL GIFT INTEGRATION
REWARD RESPONDANTS WITHOUT JEOPARDIZING ANONYMITY
Our EDC integrates with Rybbon offers best-in-class security and supports anonymous delivery, so you can offer incentives with no worries. Our Recipient Data Masking option means you can reward clinical trial participants without access to their identities.
Rewards that can be redeemed in more than 150 countries around the globe, you can incentive participants no matter where they live.

