Dacima Clinical Suite EDC software platforms offers an integrated eSource solution. The platform includes digital Direct Data Entry (DDC), electronic Patient Reported Outcomes (ePRO), electronic Clinical Outcomes Assessment (eCOA), electronic Diaries (eDiary), eConsent and other types of data collected during the trial. Data is entered into the system in real-time, eliminating the need for later transcription of data from paper copies, saving time and reducing errors.
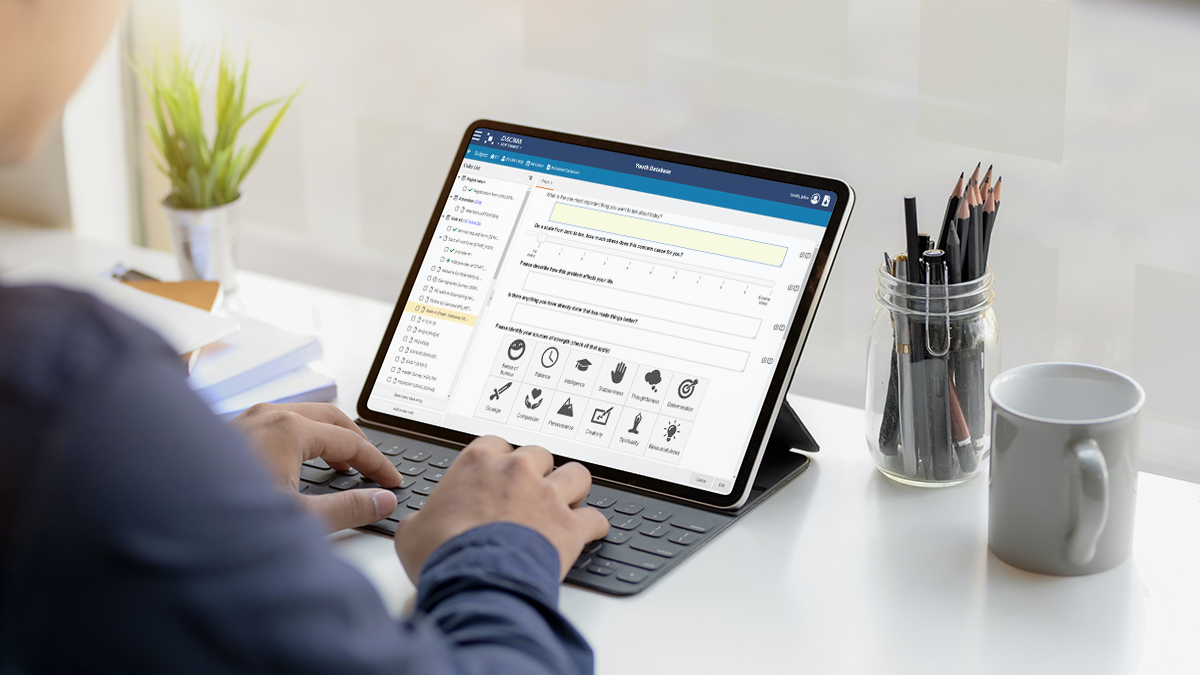
eSource in clinical studies help to:
Contact us to receive a fully functional trial version of our software including a 30-minutes guide.
@Dacima Software Inc is part of the EvidentIQ Group along with Carenity, Fortress Medical Systems and XClinical